Generate Sashimi plot using pyGenomeTracks
Sashimi plot is a powerful and popular way to visualize mRNA alternative splicing. However, there are not many tools that are designed to generate this type of plots, except for a few ones like MISO, ggSashimi and splicePlot. Moreoever, existing tools tend to have at least one of the following shortcomings: (1) tools dedicated to mRNA splicing like MISO do not allow for plotting Sashimi together with other types of genomic data, such as bigwig and bed files; (2) tools may require an annotation for extracting junctions, making them unsuitable for annotation-free methods like Leafcutter.
Motivated by these limitations, I developed a script based on the infrastructure of the Python package PyGenomeTracks. This script is not a stand-alone tool, instead it is a custom track class that is adapted from existing track classes (such as bigwig and links tracks) from PyGenomeTracks.
The scripts can be found on GitHub. To use this tool, follow the steps below:
Install PyGenomeTracks
Installation guides can be found in the original PyGenomeTracks repository or documentation.
Clone this repo to your local computer.
$ git clone https://github.com/Zepeng-Mu/pyGenomeTracks.git
Figure out where your PyGenomeTracks package is installed.
Python packages are installed in a specific folder, depending whether you used pip or conda to install the package. For example, on the high-performance computing system I’m using, the package is installed here: ~/.local/lib/python3.7/site-packages/pygenometracks/. The structure of this folder looks like:
.
├── getAllDefaultsAndPossible.py
├── __init__.py
├── makeTracksFile.py
├── plotTracks.py
├── __pycache__
│ ├── getAllDefaultsAndPossible.cpython-37.pyc
│ ├── __init__.cpython-37.pyc
│ ├── makeTracksFile.cpython-37.pyc
│ ├── plotTracks.cpython-37.pyc
│ ├── readBed.cpython-37.pyc
│ ├── readGtf.cpython-37.pyc
│ ├── tracksClass.cpython-37.pyc
│ ├── utilities.cpython-37.pyc
│ └── _version.cpython-37.pyc
├── readBed.py
├── readGtf.py
├── tracks
│ ├── BedGraphMatrixTrack.py
│ ├── BedGraphTrack.py
│ ├── BedTrack.py
│ ├── BigWigTrack.py
│ ├── EpilogosTrack.py
│ ├── GenomeTrack.py
│ ├── GtfTrack.py
│ ├── HiCMatrixTrack.py
│ ├── HLinesTrack.py
│ ├── __init__.py
│ ├── LinksTrack.py
│ ├── NarrowPeakTrack.py
│ ├── __pycache__
│ ├── ScaleBarTrack.py
│ └── TADsTrack.py
├── tracksClass.py
├── utilities.py
└── _version.py
3 directories, 35 files
The tracks directory is the key folder here. This is where all the track classes in PyGenomeTracks files are located. Track classes can only be recognized in the .ini file if the corresponding track class is located in this folder. This is also the place where we can put customized track classes. For more details see here.
Copy SashimiBigwig track class to tracks folder
Suppose you are in the folder for this cloned repo (NOT the installed PyGenomeTracks package):
cp pygenometracks/tracks/SashimiBigwig.py <PyGenomeTracks installation path>/tracks/
Prepare input files
In order to generate Sashimi plot, two types of files are needed.
- Bigwig files for RNA-seq coverage. These are just bigwig files that you would normally use for the bigwig track class.
- Link files for junctions to plot and labels. This looks like the file for the original links track class, with an additional column at the end: the number on the label (could be the number of split reads supporting the junction or PSI calculated from a certain tool.) An example file looks like this:
chr2 231109786 231109786 chr2 231110578 231110578 0.0372936854616429
chr2 231109786 231109786 chr2 231112631 231112631 0.0597340361211572
chr2 231109795 231109795 chr2 231110578 231110578 0.178226805210714
chr2 231109795 231109795 chr2 231112631 231112631 0.126256827523686
chr2 231110655 231110655 chr2 231111964 231111964 0.0149937309749814
chr2 231110655 231110655 chr2 231112631 231112631 0.195029646753571
chr2 231110655 231110655 chr2 231113600 231113600 0.0239748059030143
chr2 231112780 231112780 chr2 231113600 231113600 0.390030949667857
The column names are: chr_intron_start, pos_start, pos_start, chr_intron_end, pos_end, pos_end, psi (this is just for you to understand the file, column names should not be included in the link file).
Make a .ini file:
An example section in the .ini file:
title =
# Path to bigwig file
bw_file =
# Path to links file
link_file =
height = 0.8
bw_color =
number_of_bins =
max_value =
nans_to_zeros = true
summary_method = mean
show_data_range = true
link_color =
line_style =
fontsize = 2
# The link in Sashimi plot is a Bezier curve.
# The height of the curve is calculated from the length of the intron.
# When the y-axis in bigwig track is different, the height of curve needs to be scaled.
scale_link_height = 1
# The line width for links is proportion to the numbers at the last column in links file (PSI).
# But the absolute width is calculated from the supplied numbers, which can look too thin or too wide sometimes.
# Use scale_line_width to scale the absolute line widths.
# You may need to try several values to get a satisfying result.
scale_line_width = 3
file_type = sashimiBigWig
Run pygenometracks as you normally do to generate the figure
If you want to try the example provided, go to the cloned repo, then:
cd example_sashimi
pyGenomeTracks --tracks chr2-231091223_231109786_231113600.ini --region chr2:231107879-231115507 -t 'chr2:231109786-231113600 (sQTL = 2:231091223, ALT=G)' --width 9 --trackLabelFraction 0.01 -out example.png --fontSize 4
The resulting plot example.png looks like this: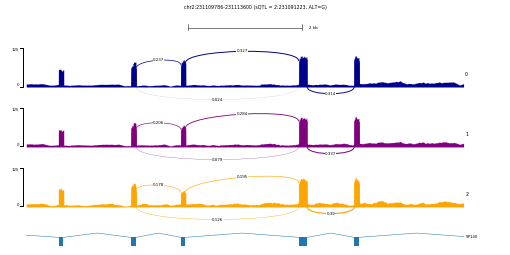
This example shows a splicing QTL (sQTL) for gene SP140. The three tracks 0, 1, and 2 represents average RNA-seq coverage for individuals with 0, 1, and 2 alternative allele (G) for SNP chr2:231091223. To generate similar plots on your data, you will need a custom script that calculate average coverage from a group of samples with the same genotype at a given position. Of course, you can also group samples by any other criteria that fits your needs, for instance combining by treatment and control.
Citation
Please refer to the original pyGenomeTracks paper:
Fidel Ramírez, Vivek Bhardwaj, Laura Arrigoni, Kin Chung Lam, Björn A. Grüning, José Villaveces, Bianca Habermann, Asifa Akhtar & Thomas Manke. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nature Communications (2018) doi:10.1038/s41467-017-02525-w